Leading the way out of stimulant addiction
(Cocaine, Amphetamines, Methamphetamines)
VANOXERINE CONSTA® (SmartDepot-Vanoxerine extended-release injection)
VANOXERINE CONSTA® must be administered under the supervision of a physician.
HIGHLIGHTS OF PRESCRIBING INFORMATION
VANOXERINE CONSTA® (Vanoxerine extended-release injection) for intramuscular use.
VANOXERINE CONSTA® should be used in conjunction with counseling and support.
INDICATIONS AND USAGE
VANOREXINE CONSTA® is indicated for use in treating cocaine dependence. VANOREXINE CONSTA® is indicated for the prevention of relapse to cocaine dependence, following cocaine detoxification process. Cocaine dependent patients must be cocaine-free at the time of initial Vanoxerine administration.
VANOREXINE CONSTA® should be part of a comprehensive management program that includes psychosocial support.
VANOREXINE CONSTA® is not a substitute for emergency medical care.
DOSAGE AND ADMINISTRATION
Due to different release characteristics, the dosage strengths are not additive and must be selected based upon the desired dosing schedule.
VANOREXINE CONSTA® 394.2 mg for 3-month administration, given as a single intramuscular injection every 12 weeks.
VANOREXINE CONSTA® 788.4 mg for 6-month administration, given as a single intramuscular injection every 24 weeks.
VANOREXINE CONSTA® 1576.8 mg for 12-month administration, given as a single intramuscular injection every 48 weeks.
The injection should be administered by a healthcare professional as an intramuscular (IM) gluteal injection, alternating buttocks for each subsequent injection. VANOREXINE CONSTA® must not be administered intravenously or subcutaneously (Avoid inadvertent administration into a blood vessel).
DOSAGE FORMS AND STRENGTHS
Injection: 394.2 mg, 788.4 mg, and 1576.8 mg Vanoxerine solution in a microsphere formulation (polyLactide-co- glycolide).
CONTRAINDICATIONS
Patients with acute hepatitis or liver failure. Patients with history of certain heart or heart valve problems (eg, mitral valve prolapse, left ventricular hypertrophy). Patients in current physiologic alcohol dependence. Any individual who are pregnant, planning to become pregnant, or are breast feeding. Patients known to be hypersensitive to Vanoxerine or are allergic to any ingredient in VANOREXINE CONSTA®.
WARNINGS AND PRECAUTIONS
Due to the duration of action, keep the patient under continued surveillance Vanoxerine may cause dizziness. These effects may be worse if you take alcohol.
ADVERSE REACTIONS
The following adverse reactions have been identified during use of Vanoxerine: back pain; diarrhea; dizziness; headache; nausea; nervousness; runny nose; stomach upset; stuffy nose; trouble sleeping, headache and nausea. These are not all side effects of the drug. This is only a summary of the most important information about the medicine. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. You are encouraged to report all side effects to the AURUM Group. Visit www.aurumgroup.org.uk or call +447535409827
DRUG INTERACTIONS
VANOREXINE CONSTA® may decrease the effectiveness of hormonal contraceptives. Patients should avoid drinking alcohol due to the duration of action VANOREXINE CONSTA®.
DESCRIPTION
VANOREXINE CONSTA® (Vanoxerine extended-release injection) for intramuscular use is supplied as a microsphere formulation of Vanoxerine. Vanoxerine is an antagonist of dopamine transporter (DAT1) with Ki value of 16.9nM. Vanoxerine, is a potent and selective dopamine reuptake inhibitor (DRI). Vanoxerine binds to the target site on the dopamine transporter (DAT) ~ 50 times more strongly than cocaine , but simultaneously inhibits the release of dopamine. This combined effect only slightly elevates dopamine levels and block the rewarding effects of cocaine.
Vanoxerine is chemically designated 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine
The molecular formula is C28H32F2N2O
The structural formula is:
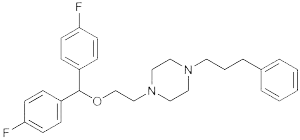
Long-Acting Injection is a combination of extended-release microspheres for injection and diluent for parenteral use. Long-Acting Injection is provided as a dose pack, consisting of a vial containing Vanoxerine microspheres and diluent.
The extended-release microspheres formulation is a white to off-white, free-flowing powder that is available in dosage strengths of 394.2 mg, 788.4 mg, or 1576.8 mg Vanoxerine per vial.
Vanoxerineis micro-encapsulated in 7011-18819 polylactide-co-glycolide (PLG) at a concentration of 372.5 mg Vanoxerine per 100 mg of microspheres.
The diluent for parenteral use is a clear, colorless solution. Composition of the diluent includes citric acid anhydrous, disodium hydrogen phosphate dihydrate, polysorbate 20, sodium carboxymethyl cellulose, sodium chloride, sodium hydroxide, and water for injection. The microspheres must be suspended in the diluent prior to injection.
SUPPLYING /STORAGE AND HANDLING
VANOREXINE CONSTA® is available in dosage strengths of 394.2 mg, 788.4 mg, or 1576.8 mg Vanoxerine. It is provided as a dose pack, consisting of a vial containing the Vanoxerine microspheres, diluent, 3 pills for Vanoxerine challenge test, Medication Guide and Directions for Use.
394.2 -mg vial/kit (ATC N04B C02-05P): 500 mg (equivalent to 394.2 mg of Vanoxerine) of a white to off-white powder provided in a vial with a rubber stopper in combination with aluminum and polypropylene cap. Each pack contains 3 vials.
788.4 -mg vial/kit (ATC N04B C02-10P): 1000 mg (equivalent to 788.4 -mg of Vanoxerine) of a white to off-white powder provided in a vial with a rubber stopper in combination with aluminum and polypropylene cap. Each pack contains 3 vials.
1576.8 -mg vial/kit (ATC N04B C02-10P): 2000 mg (equivalent to 1576.8 -mg of Vanoxerine) of a white to off-white powder provided in a vial with a rubber stopper in combination with aluminum and polypropylene cap. Each pack contains 3 vials.
Storage and Handling
The entire dose pack should be stored in the refrigerator (36°-46°F; 2°-8°C) and protected from light. If refrigeration is unavailable, can be stored at temperatures not exceeding 77°F (25°C) for no more than 7 days prior to administration. Do not expose unrefrigerated product to temperatures above 77°F (25°C)
Manufactured by:
Vanoxerine active ingredient is manufactured by: Janssen Pharmaceutica NV, Turnhoutseweg 30, Beerse, Belgium
Microspheres are manufactured by: Peptron, Inc. Biotechnology, Yuseong-gu, Daejeon, Rep. of KOREA
Diluent is manufactured by: Vetter Pharma Fertigung GmbH & Co. KG Langenargen, Germany or by S.A.I.F. S.p.a. Laboratorio Farmacologico, Cenate Sotto (Bergamo), Italy
Manufactured for Aurum Pharmaceutical Ltd
(Provided for research and clinical use only. Not for distribution or commercial use)
ESPL No: 12064/0068-3/2016